Left to right: Green car paint in sunlight vs shade—the same panels, two personalities.
Green automotive paint: Vivid outdoors and dark in shadow
January 15, 2026
Green paint blooms to life in sunlight because our vision peaks in sensitivity to green under bright light. In shade, that sensitivity drops and the finish recedes — the same car can swing from emerald to almost black, not from instability but from light and vision alone.
At noon, every leaf glimmers emerald; step into shade and those same leaves turn nearly black. Green only shows when there’s light to reflect, and car paint behaves the same way. That’s why we explain how green works — so you understand what makes it distinctive, and what to keep in mind as an owner.
Why green shifts with light
-
Nature’s lesson. Chlorophyll does double duty in plants: it absorbs red and blue light for photosynthesis, reflecting green only when light is strong. That’s why leaves look emerald in midday sun but nearly black in deep shade or at dusk.
-
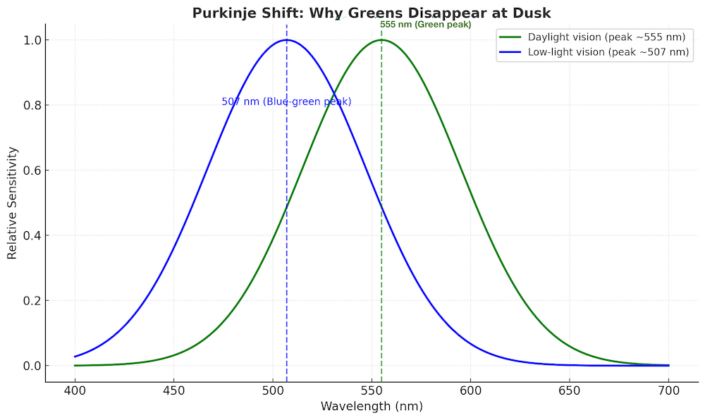
Our eyes reinforce it. Human vision peaks around ~555 nm — right in the green range — when daylight is strong. But in low light, our sensitivity shifts toward blue at ~507 nm. This is called the Purkinje shift. The effect? Deep greens fade out faster than reds or yellows as dusk falls. Grass, leaves, and green cars all seem to disappear while other colors remain visible.
-
Automotive parallels. Modern green paints often use transparent phthalocyanine pigments. Think of them like stained glass: each thin layer lets light through, stacking depth only when illumination is strong. That’s why a green test card sprayed in the lab can look thin or even bluish indoors, but outdoors it suddenly glows emerald. In sunlight, the layers show their depth; in shade, they retreat, leaving the finish subdued or nearly black.
Chart showing the Purkinje shift — under bright light, vision peaks at green (~555 nm), but at dusk it shifts toward blue (~507 nm), making greens fade faster than other colors.
The paradox of green
We live in a green world—fields, forests, chlorophyll everywhere. Yet for centuries, green was one of the hardest colors to capture in art. Malachite and verdigris paints darkened, medieval yellow–blue mixes went muddy, and 19th-century arsenic greens were vivid but toxic. The irony? The color we see most easily in nature was the trickiest to stabilize on canvas, until modern chemistry solved it with phthalo greens. That breakthrough didn’t just change art: it carried straight into automotive paint, where the same pigments define how green behaves today.
Inside the pigments that make green
That same struggle with green pigments carried over into automotive paint. Early “chrome greens” were vivid but short-lived—and made with lead chromate, a toxic pigment now heavily restricted. Mixed with Prussian blue to get that bright green, they faded quickly and posed health risks.
Today’s phthalo greens (short for phthalocyanine greens) are the safe, modern standard. They’re synthetic, lightfast, and powerful, but also transparent. That means painting with them is like working in layers of stained glass. Each thin layer builds depth, which is why the addition of every coat matters when repairing or touching up green paint.
Green paint acts like stained glass—luminous where light passes through, dark where it retreats.
Iconic greens on the road
-
Jaguar British Racing Green (1753/HGD) – Immortalized on classic Jaguars and Aston Martins. Born out of early Formula 1 regulations, it became a national identity color that still signals performance pedigree today.
-
Ford Dark Highland Green Metallic (B5/M7424A)– Steve McQueen’s Bullitt Mustang, nearly black in shade. McQueen insisted on stripping the car of badges and brightwork, making the color itself the star of the most famous chase scene in film.
-
BMW Isle of Man Green Metallic (C4G/WC4G) – A modern pearl with teal fire, a new M signature. Named after the legendary Isle of Man TT motorcycle race, the shade links BMW’s latest performance cars to speed, risk, and motorsport myth.
-
Lamborghini Verde Mantis (A3A3/A3/L0L6) – Neon-bright pearl, green at its most extroverted. Lamborghini uses “Verde” names for spectacle, and Mantis is no exception — a high-voltage hue built to be noticed on the wildest stages.
Iconic Greens on the Road — From heritage depth to neon fire, these four shades show how green paint transforms with light.
Green automotive paint — at a glance
-
Layer lightly. Expect multiple thin coats for depth, especially with transparent pigments.
-
Check in daylight. Greens look subdued indoors; confirm on a test card outdoors.
-
Expect shifts. Metallics and pearls will look darker at side angles and lighter facing the paint.
FAQ: Green automotive paint
Why does my green look almost black at night?
Our eyes lose sensitivity to green in low light, so deep greens retreat faster than other colors.
Do green paints fade faster?
Standard OEM greens are stable. Only neon or fluorescent greens fade quickly, which is why factories avoid them.
Why does my green look teal or blue under LEDs?
Light sources vary in spectrum. LEDs emphasize blue, which can tilt green finishes cooler.
Related mixing videos (green series)
Watch: Factory-accurate green mixing demonstrations from our technicians.
Toyota Cypress (6X5) — OEM Formula Color Mixing
Kawasaki Lime Green (45/777) — OEM Formula Color Mixing
Green mixing playlist: Watch the full playlist on YouTube →
Related content
Discover more stories exploring color — and the allure and armor of automotive paint.
- From the Color Series:
- Help Article: Applying Tri-Coat Layer 2, Metallic or Pearl Finish Paint – Read how metallic and pearl finishes are applied.
- Explore: Scratch Match — Compare real paint scratch photos to choose the right repair kit →
- Explore: The Color Green — Hunter Lab – Read more about the properties and history of the color green.